FloridaStateScience&
EngineeringFair

AdultRolesand
Responsibilities
• AdultSponsor
• DesignatedSupervisor
• QualifiedScientist
• IRB
• SRC

AdultSponsor
• Overseesproject
• CompletesForm1–ChecklistforAdult
Sponsor
• Usuallythescienceteacher

Designated
Supervisor
• SupervisesprojectwhenQualified
Scientistcannotdirectlysupervise
• “AnimalCareSupervisor”foranimal
projects
• SupervisesprojectsusingHazardous
Chemicals,ActivitiesorDevices,
completesandsignsForm3.

• Requiredforsomeprojects
• Doctorate/professionaldegreerelated
tostudentresearch
• CompletesForm2–QSFormand
Form3ifapplicable.
Qualified
Scientist

Researchsites
• Examplesofnon-
regulatedsites
– Home
– School
– Farm
– Ranch
– Field
– Hospitalsandclinicsmay
or/maynotbeRRI's
• Examplesofregulated
site
– IACUCReviewand
Approvalprocess
– Universities
– Governmentresearch
agencies
– Privateresearch
laboratories/hospitals

IRB
(InstitutionalReviewBoard)
• IndividualschoolscanhavetheirownIRB
• Reviewshumansubjectstudies
• Membership
– educator
– schooladministrator
– someoneknowledgeableaboutevaluatingrisk:
MD,PA,RN,psychiatrist,psychologist,licensed
socialworker(knowledgeableintheareaofthe
researchbeingevaluated)

SRC
(ScientificReviewCommittee)
• ReviewallPHBA,VertebrateandHCADprojects
BEFOREexperimentation
• Reviewallprojectsjustpriortocompetition
• Membership
– biomedicalscientist(Ph.D.,M.D.,D.V.M.,D.D.S.,
D.O.)
– scienceteacher
– othermembers
(ThesememberscannotdirectlysuperviseprojectandbeonSRC)
IntelInternational
Science&
EngineeringFair
2019
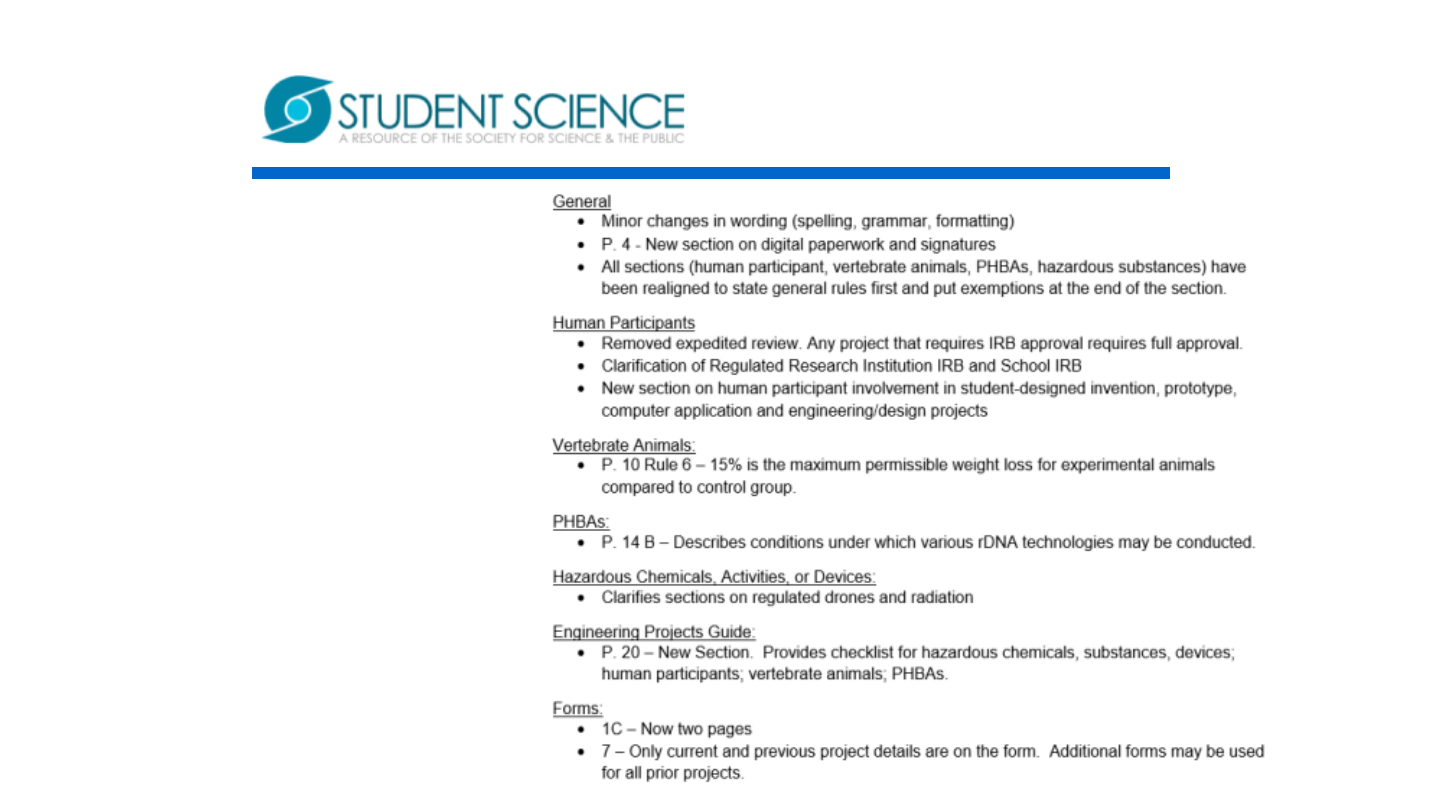
OverviewofRuleChanges
TeamsatRSEF,SSEF&ISEFwillcompeteinthe
categoryoftheirchoice.
Behavioralvertebrateprojectsareprohibitedwhich
includeoperantconditioningwithaversivestimuli.
NO BSL2 projects allowed in Junior Section.
TheuseofE.coliK-12inprojectsrequirespriorSRC
approval.NOTE:ThisisstricterthanINTELISEF
Rules/Guidelines.
RSEF/SSEF/ISEF Rule Changes
Reminders &
FloridaSSEF
Projectsinvolvingnon-humanvertebratesrequireSSEF
MortalityForm.
Anydeathwhichoccursmustbeinvestigatedbyan
individualqualifiedtodeterminethecauseofdeath,such
asaveterinarian.Theresultsoftheinvestigationmustbe
documentedinwriting.
RSEF/SSEF/ISEF Rule Changes
Reminders &
Novertebrateanimaldeathsduetotheexperimental
proceduresarepermittedinanygrouporsubgroup.Sucha
projectwillfailtoqualifyforcompetition.
FloridaSSEF
RSEF/SSEF/ISEF Rule Changes
Reminders &
FloridaSSEF
Allanimalsmustbemonitoredforsignsofdistress.
Becausesignificantweightlossisonesignof
stress,weightmustberecordedatleastweeklywith
15%beingthemaximumpermissibleweightlossor
growthretardation(comparedtocontrols)ofany
experimentalorcontrolanimal..SeePage10,#6
ISEFRules.
Studiesinvolvingthedecompositionofvertebrate
organisms(suchasinforensicprojects)requireaRisk
AssessmentForm3.
Humanandotherprimateestablishedcelllinesand
tissueculturesaretobetreatedaspotentially
hazardousbiologicalagents.Plantandnon-primate
established cell linesandtissueculturecollectionsdo
notneedtobetreatedaspotentiallyhazardous
biologicalagents.
RSEF/SSEF/ISEF Rule Changes
Reminders &
FloridaSSEF
Nobatteriesindronesatdisplay.
RSEF/SSEF/ISEF Rule Changes
Reminders &
NoQRcodesatdisplay.
Noexpeditedreviewsforhumans.
Writtenpermissionforcollectiononprivateproperty
and“managed”publiclands.
FloridaSSEF
Nobrandnames,studentproducedorcommercial
logosoracknowledgementsmaybedisplayedona
projectatRSEF/SSEF/ISEF.Thisincludesbrand
namesintheprojecttitleand/orabstract.
RSEF/SSEF/ISEF Rule Changes
Reminders &
AProjectSummaryisonlyrequiredifthestudent
makesachangeintheproceduresoutlinedinthe
student’soriginalResearchPlan.
Form1Cneededforlocationsoutsidehome,fieldor
school.Newinstructions/formonISEFForm1C.
FloridaSSEF
Theuseofwild-collectedmushroomsisprohibited.
Prohibited by SSEF
Useofcarbapenem-resistantEnterobacteriaceae
(CRE),methicillin-resistantStaphylococcusaureus
(MRSA),vancomycin-resistantEnterococci(VRE)or
KlebsiellapneumoniaCarbapenemase(KPC)
producingbacteriaandotherrelatedresistantmicrobes
isprohibited.
Sub-culturingfromMicrobialFuelCellsisprohibited
unlessworkisconductedataRegulatedResearch
Institution.
FloridaSSEF
.
Prohibited by SSEF
Noprojectinvolvingemergingpathogenscarriedby
arthropod(mosquitoes,flies,etc.)vectors
orwatersamplescollectedfromtheenvironment
containingcyanobacteriamaybeconducted
byJuniorDivisionparticipants.
Senior Division participants may conduct research on
these subjects at Registered Research Institutions
only when working with the RRI’s collection.
FloridaSSEF
.
SSEF Fair Concerns
Mentorsmustbeawareofrulesandrules
mustbeimplemented.
IRBcanbesetupbyschools
Professionalsmustbeinfieldofresearch.
Students,teachersandallinvolvedmust
becomeawareofriskswhileconducting
research.
FloridaSSEF

Form 1 is required of all
projects.
Form 1 is a fillable and
savable form
Make sure that areas
pertaining to the project
are indicated (checked).
Signatures and dates in
blue ink to indicate
“original”, not copy.
Use current year’s form!
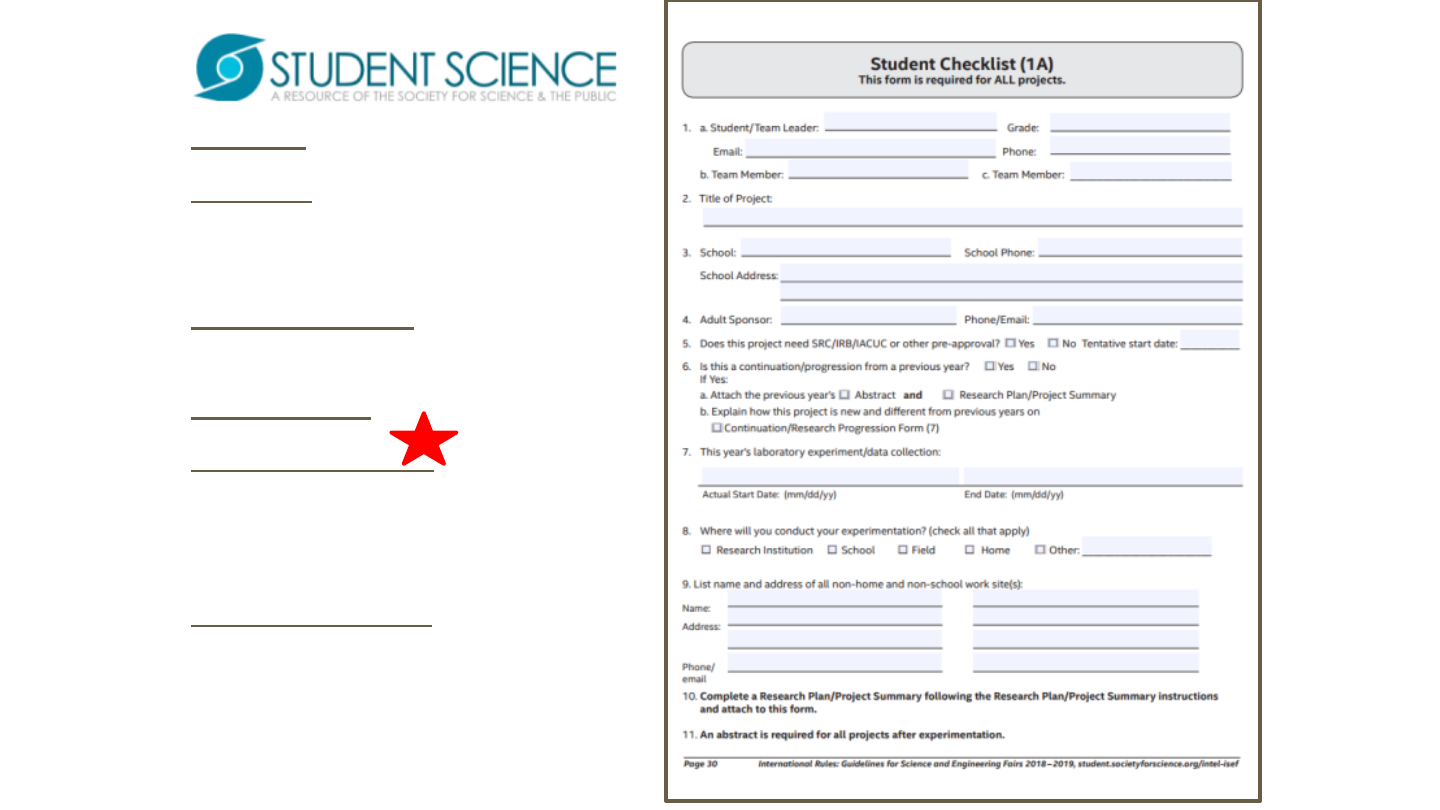
Form 1A is required of all projects.
Form 1Aisafillableandsavable
form
Start dates are for the
experimentation portion of the
project.
Florida SSEF
Husbandry projects, experimentation
date is established as the moment
you take possession of the subject
animal.
Vertebrate projects, Any action
taken involving obtaining and setting
up the experiment is included in
experimental responsibilities for the
vertebrate.

Research Plan is required of all
projects.
Rationale: Brief synopsis of the
background that supports your
research problem and explain why
this research is important scientifically.
Required items in research plan:
Human subject, vertebrate, PHBA and
HCAD projects require items listed at
right in research plan.
Data Analysis: numbered steps.
Please remember that “Data Analysis”
is not a description of how data is to
be displayed but an analysis of the
data collected.
Bibliography: Please make sure to
include required citations (depending
on nature of research). Sources
should be diverse.
Listof
requirements!

Form 1B is required of all
projects.
Form 1Bisafillableandsavable
form
Signatures and dates in blue ink
to indicate “original”, not copy.
Some projects require SRC
approval before experimentation.

Form 1C is required of projects
conducted at RRI or industrial setting.
Form 1Cisafillableandsavable
form
Pleasemakesurethatallquestions
areansweredappropriately.
Determination of requirement is
established by SRC and
rules/guidelines.
Form1Cneededforlocationsoutside
home,fieldorschool.
Signatures and dates in blue ink to
indicate “original”, not copy.
This form should be dated after the
completion of experimentation.
Newtwopage
Form!
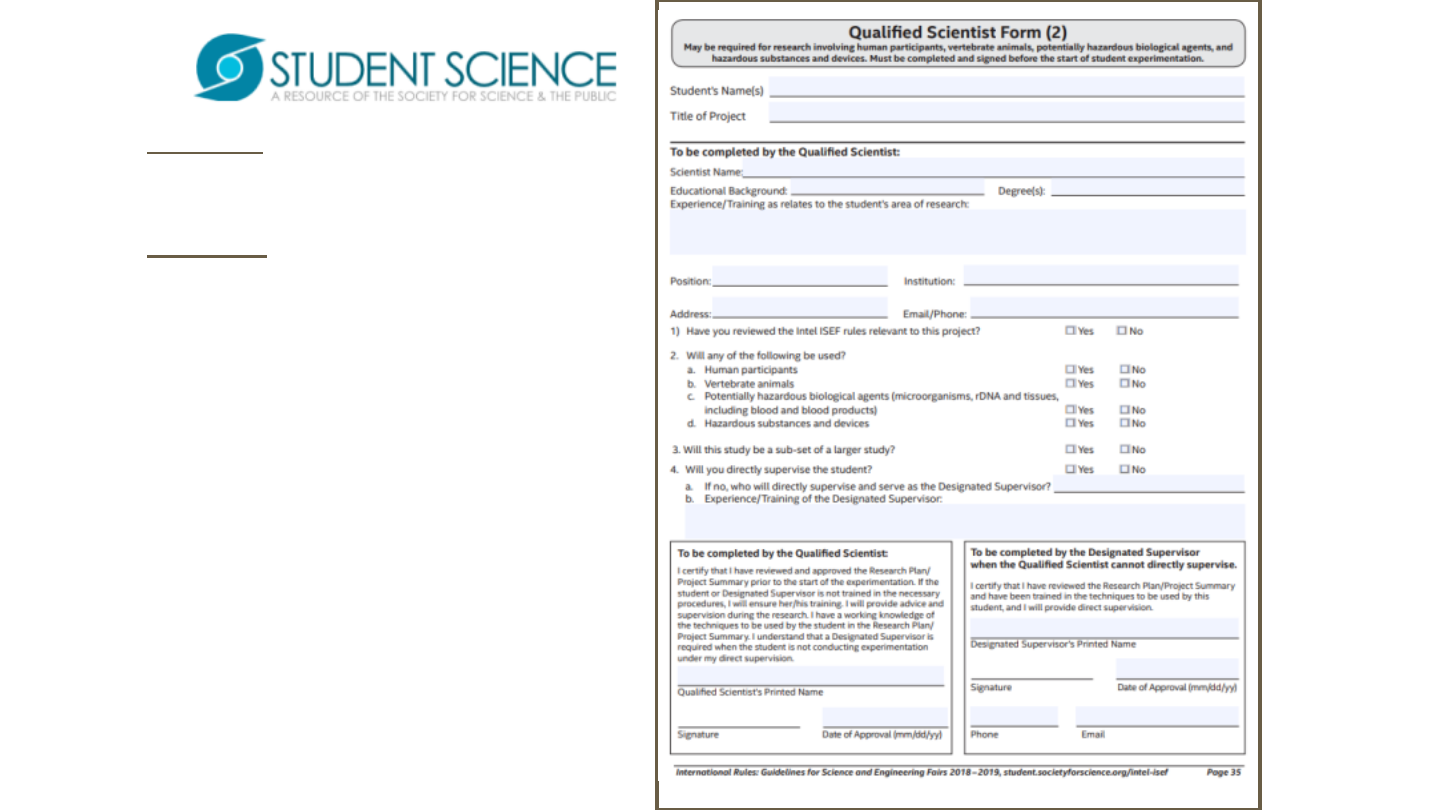
Form 2 may be required due
to the nature of research.
Form 2isafillableand
savableform.
Please make sure that all
questions are answered
appropriately.
Determination of requirement
is established by SRC/IRB and
rules/guidelines.
Signatures and dates in blue
ink to indicate “original”, not
copy.
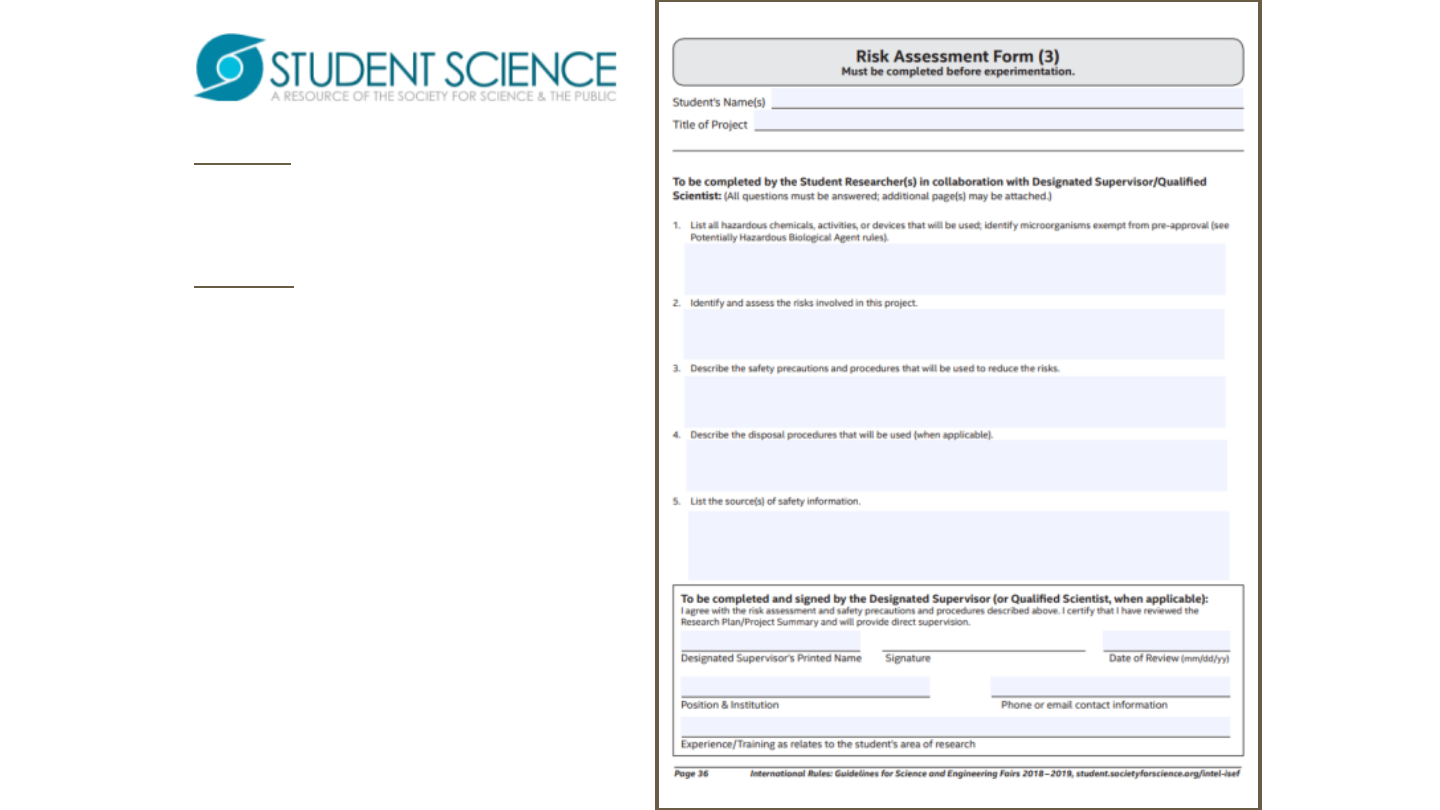
Form 3 is required of most
projects including vertebrate
animals.
Form 3isafillableandsavable
form
Pleasemakesurethatall
questionsareanswered
appropriately.
Makesuretolistappropriate
sourcesoffsafetyinformation.
Determination of requirement is
established by SRC/IRB and
rules/guidelines.
Signatures and dates in blue ink
to indicate “original”, not copy.

Form 4 is required of all projects
involving human subjects.
Form 4isafillableandsavableform.
Level of risk is determined by the
IRB.
Determination of requirement is
established by SRC/IRB and
rules/guidelines.
Florida SSEF
If a student uses humans in their
research a reference to the
protection of human subjects, MUST
be cited in their bibliography.
Signatures and dates in blue ink to
indicate “original”, not copy.
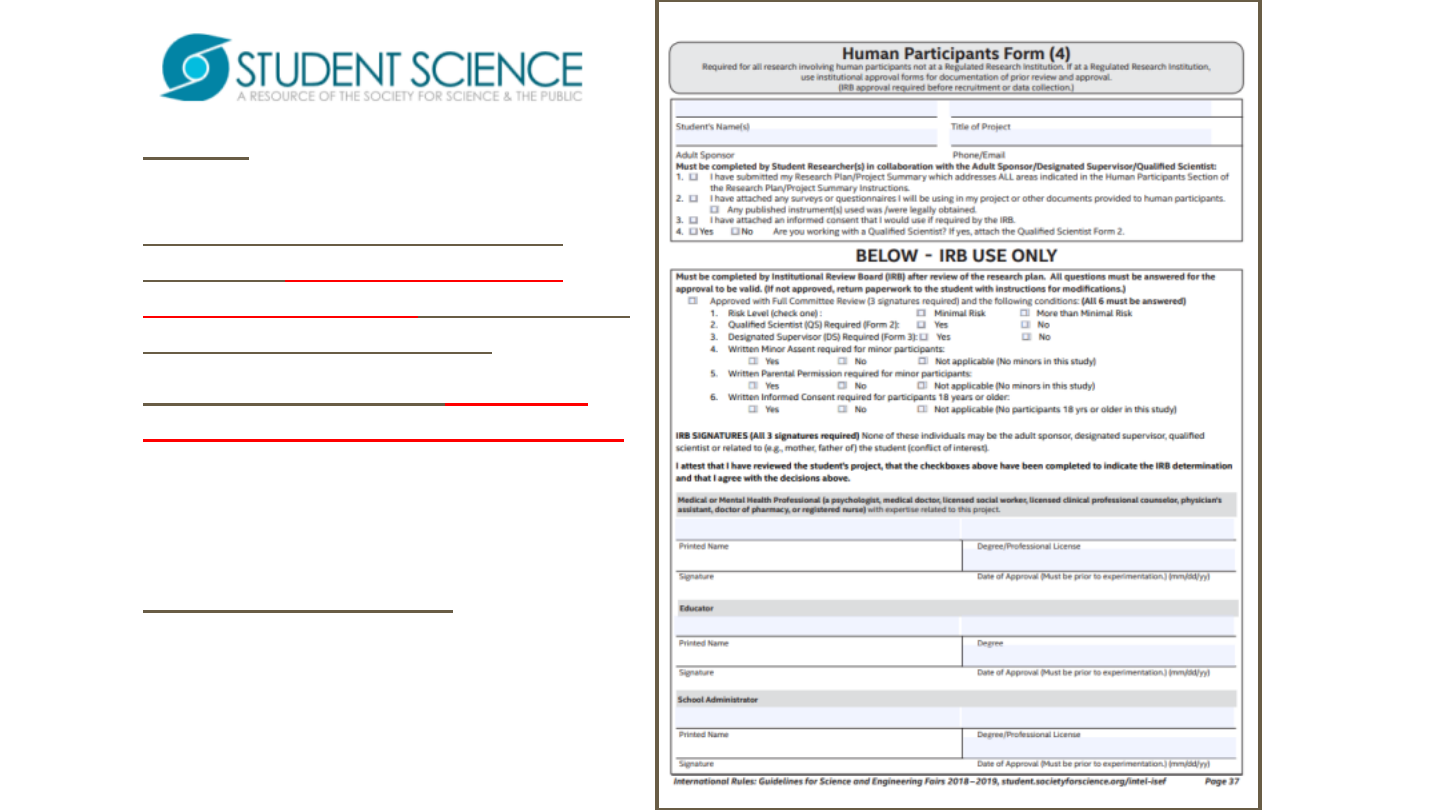
Form 4 requires review by an
Institutional Review Board (IRB).
An IRB requires at least three
members not associated with
student’s research in order to rule
out a conflict of interest.
Note that “Specialist” must have
credentials in area being reviewed.
Signatures and dates in blue ink to
indicate “original”, not copy.
Form 4 & Rule Change
An Expedited review is no longer
valid.

Informed Consent Form
is the recommended
consent form for SSEF
when IRB from Form 4
indicates this as a
required document.
Signatures and dates in
blue ink to indicate
“original”, not copy.

Human Subject Verification of
Informed Consent Form: required
form at setup prior to judging,
along with one redacted copy
(name and signature) of completed
consent form, do not redact date
and completed Form 4.
Form used to verify that
consent/assent form was obtained
from each human subject. To be
completed by adult sponsor and
student researcher after
experimentation.
Signatures and dates in blue ink to
indicate “original”, not copy.
FloridaSSEF

Form 5A is required of projects
involving vertebrates conducted at
school/home/field site.
Form 5Aisafillableandsavableform
Determination of requirement is
established by SRC and
rules/guidelines.
Required of projects involving
vertebrates at school or home.
Signatures and dates in blue ink to
indicate “original”, not copy.
If a student uses vertebrates in their
research a reference to vertebrate
subject care MUST be cited in their
bibliography.
Florida SSEF
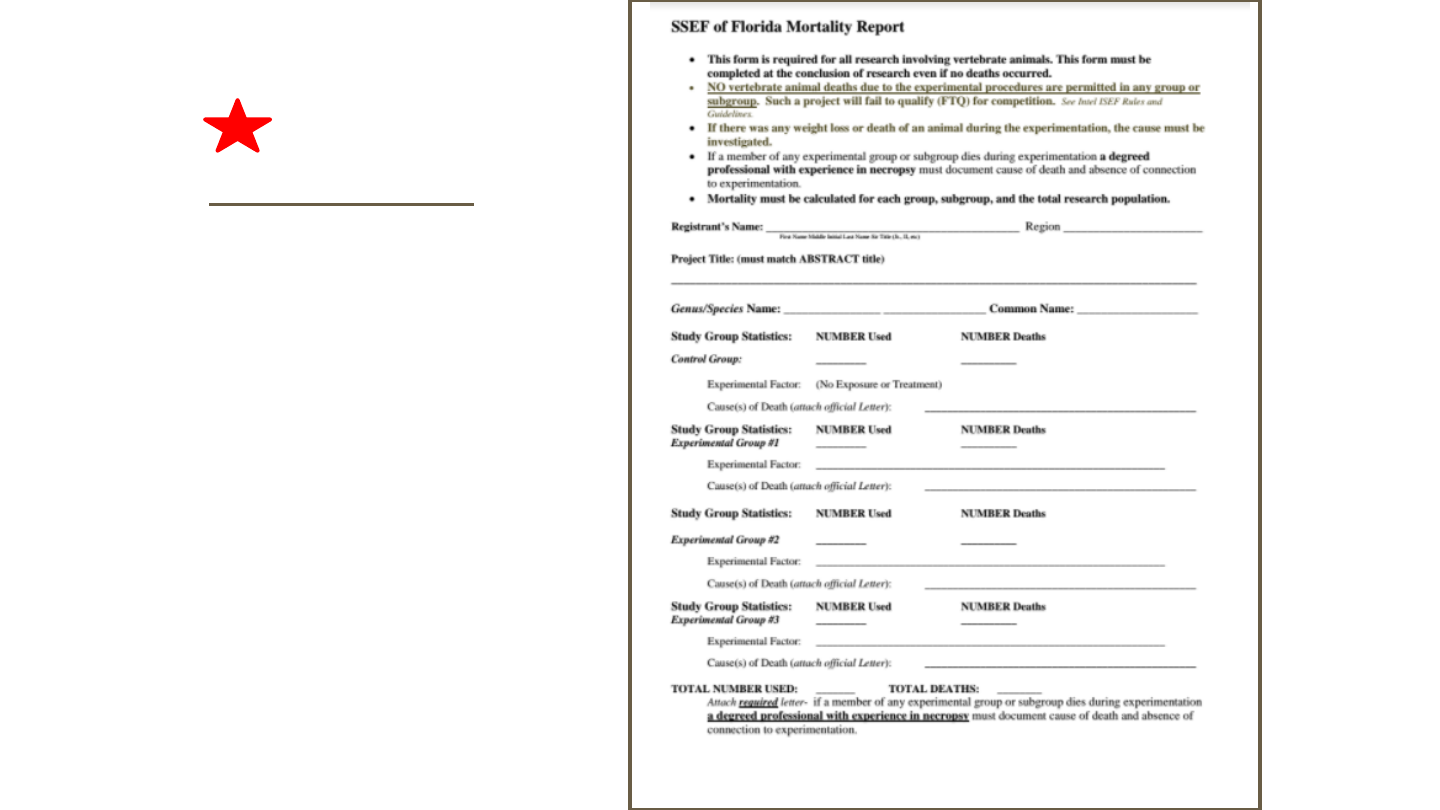
Mortality Report is
required of all projects
involving vertebrate
animals, even if no
deaths occurred.
FloridaSSEF
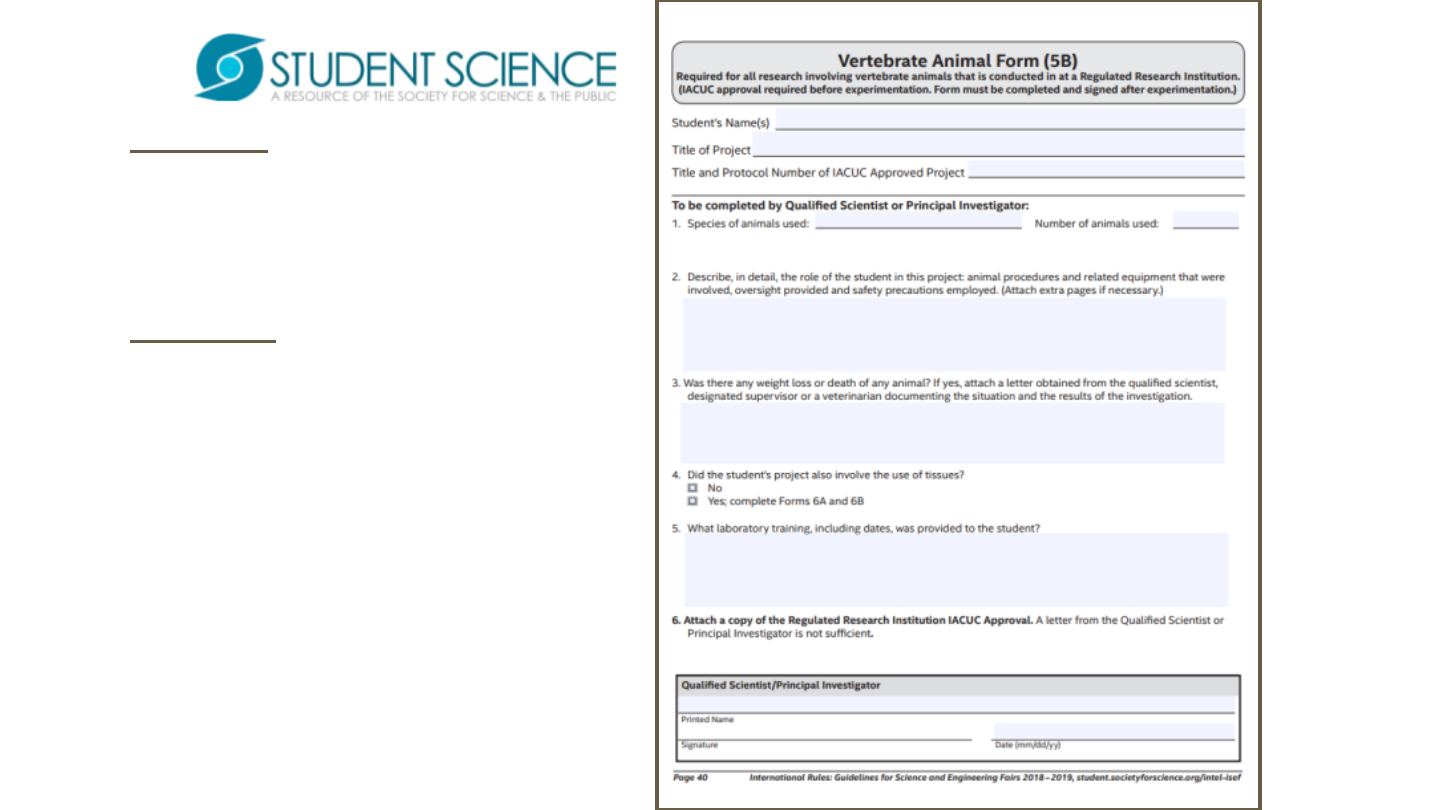
Form 5B is required of projects
involving vertebrates conducted
at Regulated Research
Institution.
Form 5Bisafillableand
savableform
Determination of requirement is
established by SRC and
rules/guidelines.
Required of projects involving
vertebrates at registered
research institutions.
Signatures and dates in blue ink
to indicate “original”, not copy.

Form 6A is required of some projects.
Form 6Aisafillableandsavableform
Level of risk must be determined,
BSL-1 or BSL-2.
Determination of requirement is
established by SRC. Determination of
BSL could be due to organisms,
procedures or both.
Signatures and dates in blue ink to
indicate “original”, not copy.
Florida SSEF
JUNIOR CATEGORY MAY NOT SUBMIT
BSL-2 PROJECTS.
If a student uses PHBAs in their
research a reference to the
appropriate microbiological technique
MUST be cited in their bibliography.

Form 6B is required of
some projects.
Form 6Bisafillableand
savableform.
Determination of
requirement is established
by SRC and
rules/guidelines.
Required of projects
involving human and
vertebrate tissue.
Signatures and dates in
blue ink to indicate
“original”, not copy.

Guidelines for Biosafety
Level 1 Laboratory
Facilities & Operations.
Required of projects
involving PHBA at a
Biosafety Level of 1.
Determined by the
completion of the risk
assessment for PHBA, Form
6A.
Signatures and dates in
blue ink to indicate
“original”, not copy.
Page ONE of 2.
PAGE1

Guidelines for Biosafety
Level 1 Laboratory
Facilities & Operations.
Required of projects
involving PHBA at a
Biosafety Level of 1.
Determined by the
completion of the risk
assessment for PHBA,
Form 6A.
Signatures and dates in
blue ink to indicate
“original”, not copy.
Page TWO of 2.
PAGE2

Guidelines for Biosafety Level
2 Laboratory Facilities &
Operations.
Required of projects involving
PHBA at a Biosafety Level of 2.
Determined by the completion
of the risk assessment for
PHBA, Form 6A.
Signatures and dates in blue
ink to indicate “original”, not
copy.
Page ONE of 3.
PAGE1
BSL2formnotrequiredfor
projectsatRegistered
ResearchInstitute.

PAGE2
Guidelines for Biosafety Level
2 Laboratory Facilities &
Operations.
Required of projects involving
PHBA at a Biosafety Level of 2.
Determined by the completion
of the risk assessment for
PHBA, Form 6A.
Signatures and dates in blue
ink to indicate “original”, not
copy.
Page TWO of 3.
BSL2formnotrequiredfor
projectsatRegistered
ResearchInstitute.

PAGE3
Guidelines for Biosafety Level
2 Laboratory Facilities &
Operations.
Required of projects involving
PHBA at a Biosafety Level of 2.
Determined by the completion
of the risk assessment for
PHBA, Form 6A.
Signatures and dates in blue
ink to indicate “original”, not
copy.
Page THREE of 3.
BSL2formnotrequiredfor
projectsatRegistered
ResearchInstitute.

Form 7 is required of some
projects.
Form 7isafillableandsavable
form
Determination of requirement is
established by SRC and
rules/guidelines.
Required of projects which are a
continuation of previous research.
Signatures and dates in blue ink
to indicate “original”, not copy.
This form can be dated after the
completion of experimentation.
NewForm!
Note …
Form 7 only
has room for
current year
and 1 (one)
previous
year… use
additional
forms for
2016-2017 or
early project
years.
Multiple
forms might
be used.

SSEF Fair Abstractis
required of all projects.
SSEF Fair Abstractisa
fillableandsavableform.
Original will be reviewed
and certified. This
certified abstract is to be
displayed vertically during
check-in and judging.
Signatures and dates in
blue ink to indicate
“original”, not copy.
FloridaSSEF

FloridaSSEFProjectCategories
SSEF Fair Categories
• ANIM 100AnimalSciences
• BEHA 200BehavioralandSocialSciences
• BMED 300BiomedicalandHealthSciences
• CMBI 400Cellular/MolecularBiology&Biochemistry
• CHEM 500Chemistry
• EAEV 600EarthandEnvironmentalSciences
• ENMS 700Engineering(EngineeringMechanics,MaterialScience,EmbeddedSystems)
• ENEV 800EnvironmentalEngineering
• IMRS 900IntelligentMachines,RoboticsandSystemsSoftware
• MACO 1000MathematicsandComputationalSciences
• MICR 1100Microbiology
• PHYS 1200Physics&Astronomy
• PLNT 1300PlantSciences
THEDEATHOFCOMMONSENSE
• Whenwearereviewingprojectsweoftenask“Didany
adultreallyreadthisbeforeapprovingtheprocedure?”
• Pleasemakesuretheprojectssubmittedadheretothe
ISEFandSSEFrules.
• Pleasedonotsubmitincompletepaperworkoraproject
thathasnotbeencritically reviewed bytheresearch
teacherbeforesubmission.
• Wearemorethanhappytoassistyouwithquestions!